Dr. Tong Mou

Fields of interest | Catalysis, photocatalytic reactions on metals and metal oxides 2D material |
| Publications | Google Scholar |
Research
My research training and experience lie in the area of atomic simulations of nanomaterials through density functional theory calculations using the VASP program. I have focused on investigating the structural, electronic and optical properties of materials, such as defects in dilute nitride alloys, functionalization of graphene and phosphorene, titanium dioxide, and vdW heterostructure. In addition, I am exploring visible-light-enhanced hydrogenation reactions on supported plasmonic metal catalysts to understand the crucial role excited electrons play in enhancing the reaction activity and selectivity for the rational design of more efficient plasmonic catalytic systems. Our group was awarded the DOE early career research grant based on my results.
Controlling reaction selectivity over hybrid plasmonic nanocatalysts
The localized surface plasmon resonance (LSPR) excitation in plasmonic nanoparticles has been used to accelerate several catalytic transformations under visible-light irradiation. In order to fully harness the potential of plasmonic catalysis, multimetallic nanoparticles containing a plasmonic and a catalytic component, where LSPR-excited energetic charge carriers and the intrinsic catalytic active sites work synergistically, have raised increased attention. Despite several exciting studies observing rate enhancements, controlling reaction selectivity remains very challenging. Here, by employing multimetallic nanoparticles combining Au, Ag, and Pt in an Au@Ag@Pt core−shell and an Au@AgPt nanorattle architectures, we demonstrate that reaction selectivity of a sequential reaction can be controlled under visible light illumination. The control of the reaction selectivity in plasmonic catalysis was demonstrated for the hydrogenation of phenylacetylene as a model transformation. We have found that the localized interaction between the triple bond in phenylacetylene and the Pt nanoparticle surface enables selective hydrogenation of the triple bond (relative to the double bond in styrene) under visible light illumination. Atomistic calculations show that the enhanced selectivity toward the partial hydrogenation product is driven by distinct adsorption configurations and charge delocalization of the reactant and the reaction intermediate at the catalyst surface.
Photoresponse of natural van der Waals heterostructures
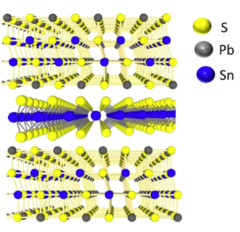
Van der Waals heterostructures consisting of 2D materials offer a platform to obtain materials by design and are very attractive owing to unique electronic states. Research on 2D van der Waals hetero-structures (vdWH) has so far been focused on fabricating individually stacked atomically thin unary or binary crystals. Such systems include graphene, hexagonal boron nitride, and members of the transition metal dichalcogenide family. Here we present our experimental study of the optoelec-tronic properties of a naturally occurring vdWH, known as franckeite, which is a complex layered crystal composed of lead, tin, antimony, iron, and sulfur. We present here that thin film franckeite (60 nm < d < 100 nm) behaves as a narrow band gap semiconductor demonstrating a wide-band photoresponse. We have observed the band-edge transition at ∼1500 nm (∼830 meV) and high external quantum efficiency (EQE ≈ 3%) at room temperature. Laser-power-resolved and temperature-resolved photocurrent measurements reveal that the photocarrier generation and recombination are dominated by continuously distributed trap states within the band gap. To understand wavelength-resolved photocurrent, we also calculated the optical absorption properties via density functional theory. Finally, we have shown that the device has a fast photoresponse with a rise time as fast as ∼1 ms. Our study provides a fundamental understanding of the optoelectronic behavior in a complex naturally occurring vdWH.
