Fundamental electronic properties of TiO2: bulk, surfaces, interfaces, nanostructures
Group of Electronic Materials: Peter Deák, Bálint Aradi, Thomas Köhler, Michael Wehlau, Moloud Kaviani, Saskia Forkert, Erik Laminski
Former members: Huy Anh Huynh, Jan Knaup, Jolla Kullgren, Jannis Ehrlich, Yannick Rodefeld, Rayna Wanbayor
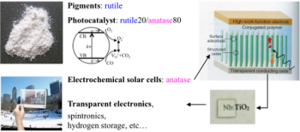
The interest in titanium oxide is rapidly growing worldwide due to its unique electronic properties, combined with the possibility of easy nanostructuring. TiO2 can in fact be manipulated by a wide spectrum of techniques and shaped into a broad range of nanoscale morphologies: particles, wires, rods, tubes and ultrathin films. This remarkable versatility has sparked interest in many fields of applications, including, electronics, spintronics, sensing, photonics, electrolysis, photo and thermocatalysis and photovoltaics. Though most of the
potential applications rely on charge carrier based mechanisms and processes, yet detailed basic research on the semiconductor nature of TiO2 is largely underrepresented, and profound theoretical understanding of related properties is still lacking. Basic research on the charge carrier generation, recombination and transport (eventually culminating in defect engineering) in single crystals, at surfaces and in nanostructures will directly benefit future design of devices.
Conventional DFT on the level of (semi-)local exchange-correlation approximations has been clearly demonstrated to fail for even qualitatively describing bulk and surface electronic properties (band gap, defect levels, localization versus delocalization behavior, electronic excitations, charge separation). Our aim is to apply a synergy of approximate and high-level theoretical methods to improve our basic understanding of the electronic processes underlying many applications.
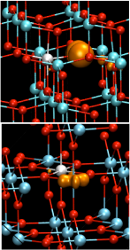
Doping of TiO2, tests of the hybrid functional
- Tests on Group-IV semiconductors and on rutile and anatase-TiO2: excellent ground state and electronic structure, linear dependence of Etot on occupation.
- Dopant passivation by electron self-trapping in rutile and hole self-trapping in anatase. Agreement with experiment.
Publications: Phys. Rev. B 81, 153203 (2010); Phys. Stat. Sol. (b) 248, 790-798 (2011); Phys. Rev. B 83, 155207 (2011);
Anatase as TCO, optical effective mass of electrons in anatase
- Calculation of the optical effective mass as a function of carrier concentration, taking into account all relevant branches of the CB gives explanation, and predicts large anisotropy. Comparison of doping with Nb and Ta.
Publications: Phys. Rev. B 83, 155201 (2011); J. Appl. Phys. 112, 016103 (2012).
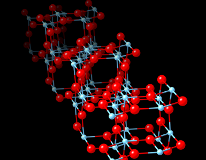
Properties of TiO2 nanowires
- Our calculations prove that NWs with a screw axis are more stable both in rutile and in anatase than without. The thinnest anatase wire with a screw axes is consistent with axes experimental results. It is observed that quantum confinement changes the relation between the gap of rutile and anatase.
- We have discovered that anatase [001] NWs with a screw axis posses massless Dirac-states, similar to graphene, but in the conduction band near to its minimum. These can be populated by a gate voltage in Ta-doped wires, switching between the classical high-resistivity semiconductor and a relativistic high-mobility range. This phenomenon could be the basis of a novel high-speed field effect transistor with high on/off ratio.
Publications: J. Phys. Chem. C, 115, 18494 (2011); Nanoletters, 13, 1073 (2013).
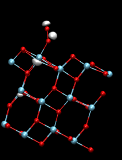
Charge assisted chemical reactions on TiO2 surfaces
- Our calculations prove that oxidation of CO can proceed spontaneously on the anatase (101) surface by the assistance of holes. We have also shown that CO preadsorption on this surface can lead to the decomposition of N2O to N2 + CO2.
Publications: J. Chem. Phys. 134, 104701 (2011); Comput. Mater. Sci. 58, 24, (2012).
Small polaron states and the properties of oxygen vacancies in the bulk and on the surface of TiO2
- We have shown that in bulk rutile the isolated oxygen vacancy automatically looses both of its electrons in favor of Ti(4+/3+) traps with bulk-like environments, which give rise to small polaron states. This does not happen at high concentrations in rutile, and ever in bulk anatase. However, small polaron states on the anatase (101) surface can trap the electrons of the vacancy. The energetically most favorable position of the vacancy in the neutral system is that of the bridging oxygen atom on the surface.
Publications: Phys. Rev. B 86, 195206 (2012); Phys. Stat. Sol. RRL, 8, 583-586 (2014).
Charge transfer between rutile and anatase
- Alignment of the perfect band structures at the charge neutrality level predicts both bands of rutile to be higher than those of anatase: electron accumulation in anatase, hole accumulation in rutile.
- Direct modelling of specific interfaces, like R(100)/A(100) and R(110)/A(101), corroborate this band alignment, in agreement with newest XPS results.
Publications: J. Phys. Chem. C, 115, 3443 (2011); Phys. Stat. Sol. RRL 8, 566-570 (2014).
